Intracranial Pressure Monitoring Devices Market Size, Trends and Insights By Technique (Invasive ICP Monitoring Devices, External Ventricular Drain (EVD) Systems, Intraparenchymal Monitors, Fiber-Optic Sensors, Micro-Transducer Systems, Subdural Monitors, Epidural Monitors, Non-Invasive ICP Monitoring Devices, Transcranial Doppler (TCD) Ultrasonography, Optic Nerve Sheath Diameter (ONSD) Measurement, Tympanic Membrane Displacement (TMD) Sensors, Pupillometry-Based Systems), By Application (Traumatic Brain Injury, Intracerebral Hemorrhage, Subarachnoid Hemorrhage, Meningitis, Hydrocephalus, Other Applications), By End-User (Hospitals, Neurosurgical Centers, Ambulatory Surgical Centers), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2026 – 2035
Report Snapshot
| Study Period: | 2026-2035 |
| Fastest Growing Market: | Asia Pacific |
| Largest Market: | North America |
Major Players
- Medtronic plc
- RAUMEDIC AG
- Sophysa Ltd.
- Spiegelberg GmbH & Co. KG
- Others
Reports Description
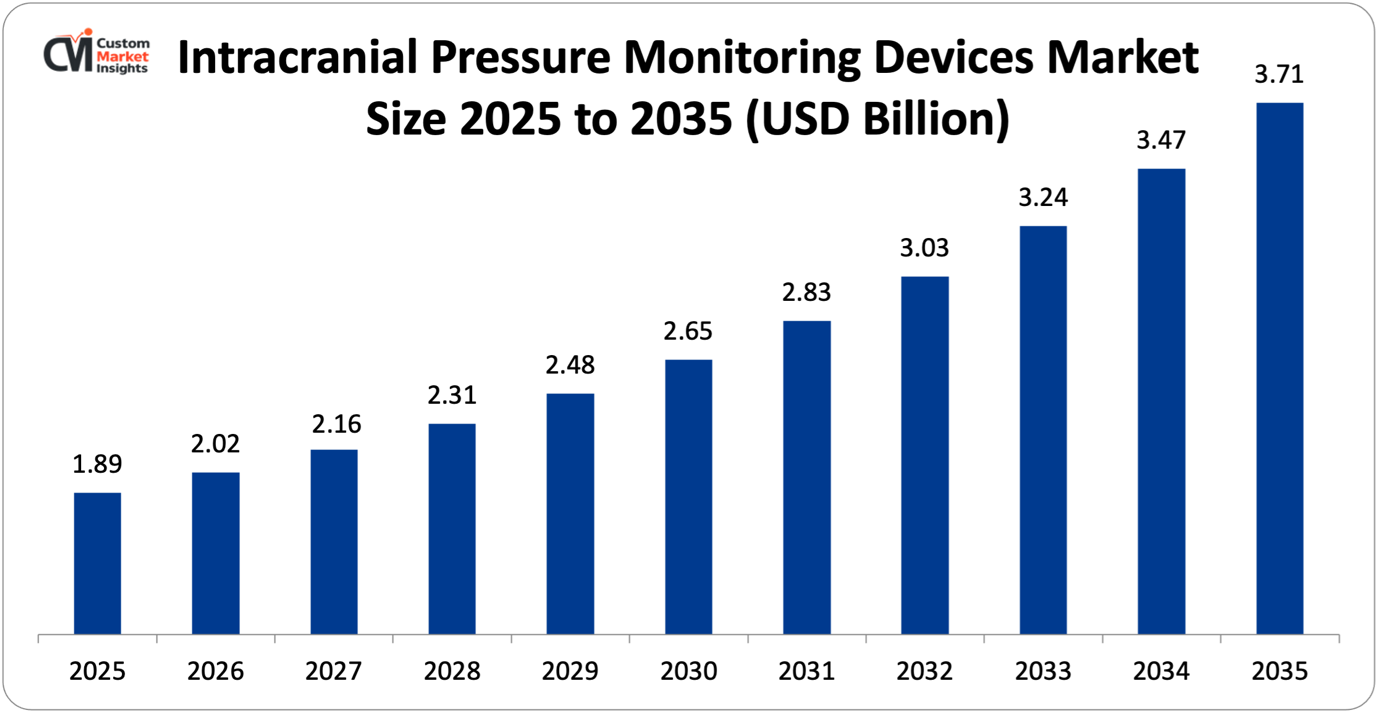
The market size of the Global Intracranial Pressure Monitoring Devices will be estimated at USD 1.89 billion in 2025 and is expected to grow between USD 2.02 billion in 2026 and about USD 3.71 billion by 2035 with a current CAGR of 7.4% during the period of 2026 to 2035. The market is growing due to the rising nature of traumatic brain injuries and neurologic diseases, the aging population, the increase in the use of minimally invasive and non-invasive monitoring technology, and the rise in the number of neurocritical care facilities.
Market Highlight
- North America had a market share of 39% market leader in the intracranial pressure monitoring devices market in 2025.
- It is projected to have the highest growth rate of 9.5% across Asia Pacific in the year 2026-2035.
- By method, the invasive ICP monitoring equipment devices slice around 74% of the market share by 2025.
- Through technology, the non-invasive ICP monitoring devices segment is increasing by a robust CAGR of 10.1% between 2026 and 2035.
- Application wise, the highest market share of 41% was attributed to the traumatic brain injury segment in 2025, and meningitis monitoring segment is projected to give 11.2% CAGR in the next period within 2026 to 2035.
- By the end-user, the hospitals segment took market share of 68% in 2025.
Intracranial Pressure Monitoring Devices Market Trends – Significant Growth Factors
The Intracranial Pressure Monitoring Devices Market Trends present significant growth opportunities due to several factors:
- Rising Prevalence of Traumatic Brain Injuries and Neurological Disorders: The prevailing trend in the intracranial pressure monitoring devices market is the rising number of traumatic brain injuries (TBI) in the world, of which the most prevalent cause of death in patients with severe head injuries is the elevated intracranial pressure. The most recent statistics released by the Center of Neuro Skills in the year 2025 reveal that the prevalence rate of traumatic brain injury is 95 per 100,000 people, and about 22% of all cases are fatal. TBI is the leading cause of death in males under the age of 35 years in the United States which has a population of 322.7 per 100, 000 individuals, and every 15 seconds, one person suffers a traumatic brain injury. A disabled population with TBI-related disability is estimated at 2% of the U.S. population today. Clinical research published in 2024 indicated the existence of over 70 million individuals with a yearly traumatic brain injury globally and the general rates of stroke are increasing especially in the younger groups of patients. The increasing incidence of nerve diseases such as stroke, intracranial bleeding, hydrocephalus, meningitis, and brain tumors develops long-term pressure measurements of the brain, which need precise measurements and continuous monitoring of the intracranial pressure. The growing number of patients that need ICP monitoring will lead to a rise in the demand of invasive and non-invasive monitoring systems, diagnostic, and treatment options, which will require the further investment in monitoring technologies and management of patients.
- Technological Advancements and Integration of AI-Powered Predictive Analytics: Technology development has significantly enhanced the market expansion because of the development of artificial intelligence and the incorporation of a neurocritical care system into the technologies. Innovations encompass wireless micro-sensors that can deliver the accuracy of 1.0 mm Hg, AI-driven predictive analytics systems capable of forecasting pressure spikes that happen up to one hour in advance with about 90% accuracy, multi-modal monitoring systems that hybridize ICP measurement including brain oxygenation and cerebral perfusion monitoring and cloud-based data management systems that allow real-time remote monitoring. By 2025, more than 60% of new ICP monitoring systems will have an embedded AI-based analytics to process real-time data and provide anomalies. New systems have become able to have continuous waveform analysis, cerebrovascular reactivity, automated warning of pressure thresholds, and smooth connection to electronic medical records. As an example, in July 2025, scholars showed that machine learning models can anticipate intracranial pressure crises in patients with severe TBI, and thus implement preventive actions before reaching critical threshold violations. In June 2024, a Chinese group developed an eight cubic millimeter ultrasonic sensor that is mini-injectable and can detect ICP wirelessly in a breakthrough in the field of minimally invasive technology. Biocompatible materials, miniaturization of sensor technologies and enhanced designs of catheters have seen medical progress that allows the monitoring to be deployed earlier, reduce the risk of infection and increase the reliability of the measurements. The innovation of continuous product development by major manufacturing companies such as Medtronic, Integra LifeSciences, and Codman Neuro keeps increasing the product portfolio and outcomes of patients who have to be strictly monitored.
What are the Major Advances Changing the Intracranial Pressure Monitoring Devices Market Today
- Non-Invasive and Minimally Invasive Monitoring Technologies: Non-invasive ICP monitoring devices development has become one of the greatest technological changes, drawing attention to pressure measurements without risks of surgery, the possibility of infections, and hemorrhage. Not invasive techniques such as transcranial Doppler ultrasonography, optic nerve sheath diameter, tympanic membrane displacement, and pupillometry techniques are now clinically acceptable at an approximation of invasive gold-standard techniques. It is reported that in the studies, the mean absolute ICP errors were about 3 mm Hg, which confirms the use of these technologies in the neurocritical care. The approval of the devices such as the i-STAT TBI cartridge by the FDA in 2024 is a case study of how the bedside diagnostics have been recognized by the regulatory body to avoid surgery. Non-invasive-monitors (NIMs) are especially useful in patients with coagulopathy, children, and in places with limited resources such as neurosurgical expertise.
- Wireless and Remote Monitoring Capabilities: The Warped patient management by the incorporation of wireless sensor technology and telemedicine platforms has transformed the ways in which patients could be managed continuously, not just in an ICU environment. Wireless micro-sensors do not require the use of heavy outer cables that can lead to infections, and this is a major benefit associated with wireless technology. In March 2025, ReFlow EVD, the novel external ventricular drain system that allows cerebrospinal fluid maintenance at home, was granted FDA Breakthrough Device Designation on Anunica Medical. Remote monitoring platforms enable experts to remotely manage the patients in several facilities, remotely access and analyze the real-time data relating to the waveform of a device, and offer expert advice to the institutions that lack the expertise on-site.
- Multi-Modal Neuromonitoring Integration: Hybrid systems of monitoring ICP and other neurophysiological parameters are capable of delivering a complete evaluation of cerebral physiology. AMTs combine ICP and brain tissue oxygen tension, cerebral blood flow, continuous EEG, and near-infrared spectroscopy. In November 2024, Nihon Kohden invested in a 71.4% stake in NeuroAdvanced, which is a major impetus to its neurological monitoring offering by special intracranial electrodes and full neuromonitoring solutions.
- Artificial Intelligence and Predictive Analytics Platforms: AI solutions are used to analyze continuous waveforms, detect increasing patterns that might signal an increase in pressure, measure cerebrovascular responsiveness, and anticipate clinical failure before conventional threshold breaches have taken place. Models of machine learning can predict ICP spikes as much as one hour before they occur with a prediction accuracy of around 90%, allowing interventions to be undertaken before brain damage is irreversible. The state-of-the-art analytics solutions facilitate automatic rejection of artifacts, adaptive monitoring of alarm thresholds, and multiparametric data integration to produce complete risk scores on which therapeutic decisions are based.
Category Wise Insights
By Technique
Why Invasive ICP Monitoring Devices Lead the Market?
In 2025 invasive monitoring technologies will be the largest of the segments representing about 74% of the market share. Invasive intracranial pressure monitoring is the best alternative, to the ongoing, precise pressure assessment in neurocritical care, and intraventricular catheters are the most precise and dependable interventions that can be used. These techniques entail sensor or catheter implantation into the brain parenchyma, ventricular system, or into the surrounding intracranial spaces and allow real time measurement of great fidelity and low drift over long monitoring intervals. In particular, clinical preference of invasive monitoring is high in severe cases of traumatic brain injury in which precise measurements are needed to make therapeutic intervention decisions.
External ventricular drain (EVD) is the most commonly used invasive method that provides two advantages of continuous ICP and therapeutic drainage of cerebrospinal fluid. Clinical data in various trauma centers show that a%age of 17.6% of severe TBI patients are monitored by ICP with the monitored showing a higher mortality rate of 31.6 than nonmonitored groups with an adjusted odds ratio of mortality of 0.44 in support of ICP monitoring.
An alternative to ventricular catheterization, which is technically difficult, is intraparenchymal monitors based on fiber-optic or micro-transducer technology. The latest intraparenchymal systems are characterized by miniaturized sensor designs, wireless telemetry as on a few models, and have the capability of measuring other parameters such as brain tissue temperature and oxygen tension.
The non-invasive ICP monitoring devices segment is growing at a very high rate with the expected CAGR of 10.1% between 2026 and 2035. Non-invasive methods also eradicate surgical risks such as hemorrhage risks, catheter malposition risks, and catheter risks. Such technologies as transcranial Doppler ultrasonography, optical nerve sheath diameter and tympanic membrane displacement sensors have reached mean absolute pressure estimation errors of about 3 mm Hg relative to invasive reference measurements. Increased clinical acceptance is especially seen in emergency department screening evaluation, patients who should not be monitored invasively and in pediatric patients, as well as in developing countries where neurosurgical skills can be inadequate.
By Application
Why Traumatic Brain Injury Dominates the ICP Monitoring Market?
The largest segment is the usage of traumatic brain injury, which has an approximation of 41% of the market share in 2025. This is in line with the clinical urgency of ICP surveillance in the severe TBI care where increased pressure is the greatest cause of death and disability. The etiology of severe TBI is directly connected to the increasing intracranial pressure, and its uncontrolled high level results in the cerebral herniation, brainstem hernia, and mortality unless timely prevention. The Brain Trauma Foundation provides specific clinical guidelines, which suggest that all salvageable individuals with severe TBI should be monitored by ICP regardless of the abnormal CT results, or those with normal CT scans and with certain clinical risk factors.
Clinical outcomes show outcome benefits in the use of pressure-guided therapy. The analysis of data of more than 10,000 severe TBI patients in 155 centers indicated that the adjusted odds ratios of mortality in the highest quartile of ICP monitoring use were lower by almost 50% than in the lowest utilization quartile, which is a significant decrease in the likelihood of death. Clinical imperative of ICP monitoring is spread over the spectrum of severity and includes monitoring of the patient with Glasgow Coma Scale between 3 and 8, decompressive craniectomy, and high risk of secondary brain injury.
One of the areas of importance applications is intracerebral hemorrhage segment, especially in cases involving patients who have large amount of hemorrhage or intraventricular extension. The spontaneous intracerebral hemorrhage is known to affect about 2 million people in the world every year, with mortality rates of the disease reaching about 40%. ICP observed allows one to note that pressure is higher because of the hematoma expansion, perihematomal edema, or obstructive hydrocephalus.
The subarachnoid hemorrhage segment makes use of ICP monitoring widely in the acute treatment of aneurysmal rupture, and extravascular ventricular drain insertion to simultaneously measure the pressure and provide therapeutic CSF debridement. In the United States, aneurysmal subarachnoid hemorrhage befalls about 30,000 individuals annually.
The meningitis surveillance segment is steadily recording the highest growth at a projected CAGR of 11.2% in 2026-2035, as there is growing awareness on the fact that infectious meningitis may cause life-threatening intracranial hypertension, which should be monitored and treated aggressively.
By End-User
Why Hospitals Dominate ICP Monitoring Device Adoption?
Hospitals will have the highest market share end user segment of around 68% in 2025. The only extreme neurological cases that do need ICP monitoring are those that are under the care of the hospital at least within the intensive care unit which has special neurocritical care facilities and that have direct access to the neurosurgical services. The main users of the ICP monitoring technology are Level I and Level II trauma centers, extensive stroke centers, and hospitals with a specialized neurointensive care unit.
In hospital systems, intensive care units are the main focal point of deploying ICP monitoring, and neurointensive care units are the core of the advanced neuromonitoring practices. The teams staffing such specialized ICU facilities include neurocritical care physicians, neurosurgeons, specialized nursing staff as well as respiratory staff with experience in handling brain injured patients. As per the healthcare infrastructure statistics, in 2024, hospitals in North America spent USD 18 billion on new monitoring infrastructure, and significant proportions of money were assigned to the neurocritical care capability development.
Another key user group is the emergency departments in trauma centers, as ICP monitoring is being increasingly performed in emergency departments in patients with severe TBI. The pattern of earlier initiation of monitoring is a clinical finding that delayed identification of intracranial hypertension is a risk factor.
A developing branch is that of neurosurgical centers and specialized neurological hospitals, which in most cases provide regional referral centers to complex neurosurgical cases. There are some academic medical centers that have neuroscience intensive care units that handle 200-300 ICP monitor patients every year, which is a significant use of the equipment.
Ambulatory surgical centers are a new segment with the current usage being minimal since the acuity of the condition needing ICP monitoring is high. Yet, it can be seen that future growth opportunities towards lower-acuity settings might be fostered by the creation of more advanced monitoring systems such as wireless sensors and remote monitoring systems.
Report Scope
| Feature of the Report | Details |
| Market Size in 2026 | USD 2.02 billion |
| Projected Market Size in 2035 | USD 3.71 billion |
| Market Size in 2025 | USD 1.89 billion |
| CAGR Growth Rate | 7.4% CAGR |
| Base Year | 2025 |
| Forecast Period | 2026-2035 |
| Key Segment | By Technique, Application, End-User and Region |
| Report Coverage | Revenue Estimation and Forecast, Company Profile, Competitive Landscape, Growth Factors and Recent Trends |
| Regional Scope | North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America |
| Buying Options | Request tailored purchasing options to fulfil your requirements for research. |
Intracranial Pressure Monitoring Devices Market – Regional Analysis
How Big is the North America Intracranial Pressure Monitoring Devices Market Size?
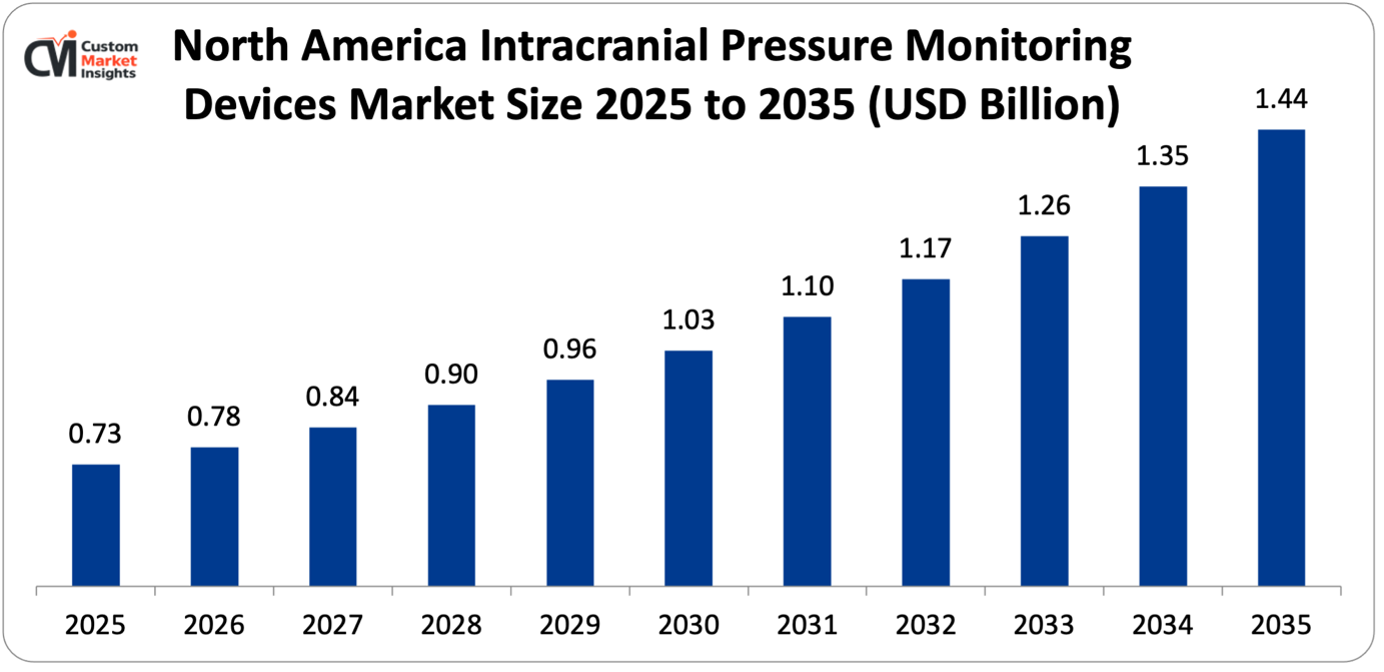
Its market size, in terms of North America intracranial pressure monitoring devices, is projected to be USD 737 million in 2025 with a growth of about USD 1,446 million in 2035 with a CAGR of 7.2% between 2026 and 2035.
Why did North America Dominate the ICP Monitoring Devices Market in 2025?
In 2025, North America will dominate the global market with an estimated market share of 39% due to the well-developed trauma care systems, well-established neurocritical care systems, high rates of traumatic brain injuries, robust reimbursement systems, and the existence of the leading device manufacturers. United States shows high rates of adoption of ICP monitoring due to clinical recommendations by agencies such as the Brain Trauma Foundation, reimbursement provided by Medicare and commercial insurers, and legal aspects promoting the use of extensive monitoring in case of severe neurological injuries.
What is the Size of the U.S. Intracranial Pressure Monitoring Devices Market?
The market size of the U.S. intracranial pressure monitoring devices is estimated to be USD 635 million in 2025 and USD 1,245 million in 2035, and growing at a good rate of 7.1% in the period between 2026 and 2035.
U.S. Intracranial Pressure Monitoring Devices Market Trends
The US market has the most significant share of the worldwide demand due to high rates of traumatic brain injury (TBI is a leading cause of death among injuries), extensive facilities of state-of-the-art neurosurgical services, the ongoing technological development, positive reimbursement conditions, and the growing use of multimodal neuromonitoring methods. Innovation and clinical adoption is further stimulated by the U.S. military emphasis on the management of TBI, including baseline cognitive testing of all new Army recruits since June 2024 and heavy investment in technologies to monitor the consequences of blast injuries.
Why is Asia Pacific Experiencing the Fastest Growth in the ICP Monitoring Devices Market?
It is estimated that the Asia-Pacific region will have the highest growth with a CAGR of 9.5 between the year 2026 and 2035. The high rate of road traffic accidents, rising occurrence of stroke and cerebrovascular diseases due to aging population, extensive government funding in health care infrastructure, augmented awareness about the international excellence in neurocritical care, and augmented expenditure in the medical sector, which follows with the development of the economy are the factors that testify to rapid growth of traumatic brain injury incidence.
China Intracranial Pressure Monitoring Devices Market Trends
The Chinese market has an extremely fast growing market due to government efforts to reform healthcare, the large investments in infrastructure building new hospitals and new ICUs, the growing number of trained neurosurgeons, the increasing medical device regulatory sophistication, and the expectation of patients. An example of indigenous innovation leadership is a 2024 Chinese technology breakthrough of mini-injectable ultrasonic sensors, making China one of the leading market players and a new source of technology.
Why is Europe Entering a New Era of Intracranial Pressure Monitoring Market?
The European market is large and established, which indicates the presence of well-developed healthcare systems, well-developed neurosurgical training programs, well-developed trauma care networks, and high attention paid to evidence-based clinical practice. Europe still has a large market share in the world with Germany, United Kingdom, France, Italy and Spain being some of the key consumers. The European clinical practice has high compliance with international guidelines and advanced neurocritical care facilities.
Germany Intracranial Pressure Monitoring Devices Market Trends
The high-developed healthcare system, large number of trauma centers and university hospitals, high level of neurosurgical experience, and full coverage of the healthcare insurance system in favor of the well-developed monitoring technologies predetermine the presence of the largest ICP monitoring markets in Germany.
Why is the Middle East & Africa Region Accelerating Adoption of ICP Monitoring Devices?
The market development is diverse with a high degree of heterogeneity among countries in the LAMEA area. The Middle East, and especially GCC countries such as Saudi Arabia and UAE are showing a growing adoption due to significant investments in healthcare infrastructure, the creation of specialized neuroscience centers, and the desire to create the regional centers of medical excellence. Africa is more nascent in the development of market, but South Africa in its features, has more sophisticated capabilities with well-developed neurosurgical programs in major academic hospitals.
Brazil Intracranial Pressure Monitoring Devices Market Trends
The market development of Brazil is linked to the development of the private healthcare sector, the rise of the access to neurosurgical care in large cities, the expansion of the use of foreign clinical practices, and the rising awareness of traumatic brain injury as a significant health issue in society. With the expansion of the Brazilian healthcare access, as well as the neurosurgical workforce, the future of the market development is optimistic.
Top Players in the Intracranial Pressure Monitoring Devices Market and Their Offerings
- Medtronic plc
- Integra LifeSciences Holdings Corporation
- Codman & Shurtleff Inc. (Johnson & Johnson)
- Natus Medical Incorporated
- RAUMEDIC AG
- Sophysa Ltd.
- Spiegelberg GmbH & Co. KG
- Vittamed
- Nihon Kohden Corporation
- Compumedics Limited
- Others
Intracranial Pressure Monitoring Devices Market News- Key Developments
Intracranial Pressure Monitoring Devices Market has experienced considerable changes in the last two years as the market players are trying to diversify their technological aspects and develop product portfolio using strategic approaches.
- In November 2024: Nihon Kohden Corporation purchased another company, Ad-Tech Medical Instrument Corporation, which owns NeuroAdvanced; the parent company bought 71.4% of the company. The depth and subdural electrode, EEG monitoring of the neurology and comprehensive neuromonitoring solutions are also added in this strategic acquisition that has greatly empowered the Nihon Kohden to monitor using specialized intracranial electrode and depth monitoring as well as subdural monitoring. (Source: https://www.nihonkohden.com/)
- In March 2025: Anunica Medical shared that its ReFlow EVD system was given Breakthrough Designation of FDA. ReFlow EVD system allows at home maintenance of cerebral spinal fluid in patients who need chronic CSF diversion which may help prevent the need to undergo revision surgeries and improve the quality of life of patients with hydrocephalus. (Source: https://www.anunciamed.com/)
These strategic measures have enabled the companies to reinforce their competitive positions, increase the product line, boost their technological competencies and also seize growth opportunities in the fast growing Intracranial Pressure Monitoring Devices Market.
The Intracranial Pressure Monitoring Devices Market is segmented as follows:
By Technique
- Invasive ICP Monitoring Devices
- External Ventricular Drain (EVD) Systems
- Intraparenchymal Monitors
- Fiber-Optic Sensors
- Micro-Transducer Systems
- Subdural Monitors
- Epidural Monitors
- Non-Invasive ICP Monitoring Devices
- Transcranial Doppler (TCD) Ultrasonography
- Optic Nerve Sheath Diameter (ONSD) Measurement
- Tympanic Membrane Displacement (TMD) Sensors
- Pupillometry-Based Systems
By Application
- Traumatic Brain Injury
- Intracerebral Hemorrhage
- Subarachnoid Hemorrhage
- Meningitis
- Hydrocephalus
- Other Applications
By End-User
- Hospitals
- Neurosurgical Centers
- Ambulatory Surgical Centers
Regional Coverage:
North America
- U.S.
- Canada
- Mexico
- Rest of North America
Europe
- Germany
- France
- U.K.
- Russia
- Italy
- Spain
- Netherlands
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Taiwan
- Rest of Asia Pacific
The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America
Table of Contents
- Chapter 1. Preface
- 1.1 Report Description and Scope
- 1.2 Research scope
- 1.3 Research methodology
- 1.3.1 Market Research Type
- 1.3.2 Market research methodology
- Chapter 2. Executive Summary
- 2.1 Global Intracranial Pressure Monitoring Devices Market, (2026 – 2035) (USD Billion)
- 2.2 Global Intracranial Pressure Monitoring Devices Market : snapshot
- Chapter 3. Global Intracranial Pressure Monitoring Devices Market – Industry Analysis
- 3.1 Intracranial Pressure Monitoring Devices Market: Market Dynamics
- 3.2 Market Drivers
- 3.2.1 Growing prevalence of traumatic brain injuries and neurological diseases
- 3.2.2 Growing geriatric population with a higher rate of stroke
- 3.2.3 Growing popularity of less invasive and non-invasive monitoring devices
- 3.2.4 Growing number of neurocritical care facilities globally
- 3.3 Market Restraints
- 3.4 Market Opportunities
- 3.5 Market Challenges
- 3.6 Porter’s Five Forces Analysis
- 3.7 Market Attractiveness Analysis
- 3.7.1 Market attractiveness analysis By Technique
- 3.7.2 Market attractiveness analysis By Application
- 3.7.3 Market attractiveness analysis By End-User
- Chapter 4. Global Intracranial Pressure Monitoring Devices Market- Competitive Landscape
- 4.1 Company market share analysis
- 4.1.1 Global Intracranial Pressure Monitoring Devices Market: company market share, 2025
- 4.2 Strategic development
- 4.2.1 Acquisitions & mergers
- 4.2.2 New Product launches
- 4.2.3 Agreements, partnerships, cullaborations, and joint ventures
- 4.2.4 Research and development and Regional expansion
- 4.3 Price trend analysis
- 4.1 Company market share analysis
- Chapter 5. Global Intracranial Pressure Monitoring Devices Market – Technique Analysis
- 5.1 Global Intracranial Pressure Monitoring Devices Market overview: By Technique
- 5.1.1 Global Intracranial Pressure Monitoring Devices Market share, By Technique, 2025 and 2035
- 5.2 Invasive ICP Monitoring Devices
- 5.2.1 Global Intracranial Pressure Monitoring Devices Market by Invasive ICP Monitoring Devices, 2026 – 2035 (USD Billion)
- 5.3 External Ventricular Drain (EVD) Systems
- 5.3.1 Global Intracranial Pressure Monitoring Devices Market by External Ventricular Drain (EVD) Systems, 2026 – 2035 (USD Billion)
- 5.4 Intraparenchymal Monitors
- 5.4.1 Global Intracranial Pressure Monitoring Devices Market by Intraparenchymal Monitors , 2026 – 2035 (USD Billion)
- 5.5 Fiber-Optic Sensors
- 5.5.1 Global Intracranial Pressure Monitoring Devices Market by Fiber-Optic Sensors, 2026 – 2035 (USD Billion)
- 5.6 Micro-Transducer Systems
- 5.6.1 Global Intracranial Pressure Monitoring Devices Market by Micro-Transducer Systems, 2026 – 2035 (USD Billion)
- 5.7 Subdural Monitors
- 5.7.1 Global Intracranial Pressure Monitoring Devices Market by Subdural Monitors, 2026 – 2035 (USD Billion)
- 5.8 Epidural Monitors
- 5.8.1 Global Intracranial Pressure Monitoring Devices Market by Epidural Monitors, 2026 – 2035 (USD Billion)
- 5.9 Non-Invasive ICP Monitoring Devices
- 5.9.1 Global Intracranial Pressure Monitoring Devices Market by Non-Invasive ICP Monitoring Devices, 2026 – 2035 (USD Billion)
- 5.10 Transcranial Doppler (TCD) Ultrasonography
- 5.10.1 Global Intracranial Pressure Monitoring Devices Market by Transcranial Doppler (TCD) Ultrasonography, 2026 – 2035 (USD Billion)
- 5.11 Optic Nerve Sheath Diameter (ONSD) Measurement
- 5.11.1 Global Intracranial Pressure Monitoring Devices Market by Optic Nerve Sheath Diameter (ONSD) Measurement, 2026 – 2035 (USD Billion)
- 5.12 Tympanic Membrane Displacement (TMD) Sensors
- 5.12.1 Global Intracranial Pressure Monitoring Devices Market by Tympanic Membrane Displacement (TMD) Sensors, 2026 – 2035 (USD Billion)
- 5.13 Pupillometry-Based Systems
- 5.13.1 Global Intracranial Pressure Monitoring Devices Market by Pupillometry-Based Systems, 2026 – 2035 (USD Billion)
- 5.1 Global Intracranial Pressure Monitoring Devices Market overview: By Technique
- Chapter 6. Global Intracranial Pressure Monitoring Devices Market – Application Analysis
- 6.1 Global Intracranial Pressure Monitoring Devices Market overview: By Application
- 6.1.1 Global Intracranial Pressure Monitoring Devices Market share, By Application, 2025 and 2035
- 6.2 Traumatic Brain Injury
- 6.2.1 Global Intracranial Pressure Monitoring Devices Market by Traumatic Brain Injury, 2026 – 2035 (USD Billion)
- 6.3 Intracerebral Hemorrhage
- 6.3.1 Global Intracranial Pressure Monitoring Devices Market by Intracerebral Hemorrhage, 2026 – 2035 (USD Billion)
- 6.4 Subarachnoid Hemorrhage
- 6.4.1 Global Intracranial Pressure Monitoring Devices Market by Subarachnoid Hemorrhage, 2026 – 2035 (USD Billion)
- 6.5 Meningitis
- 6.5.1 Global Intracranial Pressure Monitoring Devices Market by Meningitis, 2026 – 2035 (USD Billion)
- 6.6 Hydrocephalus
- 6.6.1 Global Intracranial Pressure Monitoring Devices Market by Hydrocephalus, 2026 – 2035 (USD Billion)
- 6.7 Other Applications
- 6.7.1 Global Intracranial Pressure Monitoring Devices Market by Other Applications, 2026 – 2035 (USD Billion)
- 6.1 Global Intracranial Pressure Monitoring Devices Market overview: By Application
- Chapter 7. Global Intracranial Pressure Monitoring Devices Market – End-User Analysis
- 7.1 Global Intracranial Pressure Monitoring Devices Market overview: By End-User
- 7.1.1 Global Intracranial Pressure Monitoring Devices Market share, By End-User, 2025 and 2035
- 7.2 Hospitals
- 7.2.1 Global Intracranial Pressure Monitoring Devices Market by Hospitals, 2026 – 2035 (USD Billion)
- 7.3 Neurosurgical Centers
- 7.3.1 Global Intracranial Pressure Monitoring Devices Market by Neurosurgical Centers, 2026 – 2035 (USD Billion)
- 7.4 Ambulatory Surgical Centers
- 7.4.1 Global Intracranial Pressure Monitoring Devices Market by Ambulatory Surgical Centers, 2026 – 2035 (USD Billion)
- 7.1 Global Intracranial Pressure Monitoring Devices Market overview: By End-User
- Chapter 8. Intracranial Pressure Monitoring Devices Market – Regional Analysis
- 8.1 Global Intracranial Pressure Monitoring Devices Market Regional Overview
- 8.2 Global Intracranial Pressure Monitoring Devices Market Share, by Region, 2025 & 2035 (USD Billion)
- 8.3. North America
- 8.3.1 North America Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.3.1.1 North America Intracranial Pressure Monitoring Devices Market, by Country, 2026 – 2035 (USD Billion)
- 8.3.1 North America Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.4 North America Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035
- 8.4.1 North America Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035 (USD Billion)
- 8.5 North America Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035
- 8.5.1 North America Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035 (USD Billion)
- 8.6 North America Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035
- 8.6.1 North America Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035 (USD Billion)
- 8.7. Europe
- 8.7.1 Europe Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.7.1.1 Europe Intracranial Pressure Monitoring Devices Market, by Country, 2026 – 2035 (USD Billion)
- 8.7.1 Europe Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.8 Europe Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035
- 8.8.1 Europe Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035 (USD Billion)
- 8.9 Europe Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035
- 8.9.1 Europe Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035 (USD Billion)
- 8.10 Europe Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035
- 8.10.1 Europe Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035 (USD Billion)
- 8.11. Asia Pacific
- 8.11.1 Asia Pacific Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.11.1.1 Asia Pacific Intracranial Pressure Monitoring Devices Market, by Country, 2026 – 2035 (USD Billion)
- 8.11.1 Asia Pacific Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.12 Asia Pacific Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035
- 8.12.1 Asia Pacific Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035 (USD Billion)
- 8.13 Asia Pacific Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035
- 8.13.1 Asia Pacific Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035 (USD Billion)
- 8.14 Asia Pacific Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035
- 8.14.1 Asia Pacific Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035 (USD Billion)
- 8.15. Latin America
- 8.15.1 Latin America Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.15.1.1 Latin America Intracranial Pressure Monitoring Devices Market, by Country, 2026 – 2035 (USD Billion)
- 8.15.1 Latin America Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.16 Latin America Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035
- 8.16.1 Latin America Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035 (USD Billion)
- 8.17 Latin America Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035
- 8.17.1 Latin America Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035 (USD Billion)
- 8.18 Latin America Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035
- 8.18.1 Latin America Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035 (USD Billion)
- 8.19. The Middle-East and Africa
- 8.19.1 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.19.1.1 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by Country, 2026 – 2035 (USD Billion)
- 8.19.1 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, 2026 – 2035 (USD Billion)
- 8.20 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035
- 8.20.1 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by Technique, 2026 – 2035 (USD Billion)
- 8.21 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035
- 8.21.1 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by Application, 2026 – 2035 (USD Billion)
- 8.22 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035
- 8.22.1 The Middle-East and Africa Intracranial Pressure Monitoring Devices Market, by End-User, 2026 – 2035 (USD Billion)
- Chapter 9. Company Profiles
- 9.1 Medtronic plc
- 9.1.1 Overview
- 9.1.2 Financials
- 9.1.3 Product Portfolio
- 9.1.4 Business Strategy
- 9.1.5 Recent Developments
- 9.2 Integra LifeSciences Holdings Corporation
- 9.2.1 Overview
- 9.2.2 Financials
- 9.2.3 Product Portfolio
- 9.2.4 Business Strategy
- 9.2.5 Recent Developments
- 9.3 Codman & Shurtleff Inc. (Johnson & Johnson)
- 9.3.1 Overview
- 9.3.2 Financials
- 9.3.3 Product Portfolio
- 9.3.4 Business Strategy
- 9.3.5 Recent Developments
- 9.4 Compumedics Limited
- 9.4.1 Overview
- 9.4.2 Financials
- 9.4.3 Product Portfolio
- 9.4.4 Business Strategy
- 9.4.5 Recent Developments
- 9.5 Natus Medical Incorporated
- 9.5.1 Overview
- 9.5.2 Financials
- 9.5.3 Product Portfolio
- 9.5.4 Business Strategy
- 9.5.5 Recent Developments
- 9.6 RAUMEDIC AG
- 9.6.1 Overview
- 9.6.2 Financials
- 9.6.3 Product Portfolio
- 9.6.4 Business Strategy
- 9.6.5 Recent Developments
- 9.7 Sophysa Ltd.
- 9.7.1 Overview
- 9.7.2 Financials
- 9.7.3 Product Portfolio
- 9.7.4 Business Strategy
- 9.7.5 Recent Developments
- 9.8 Spiegelberg GmbH & Co. KG
- 9.8.1 Overview
- 9.8.2 Financials
- 9.8.3 Product Portfolio
- 9.8.4 Business Strategy
- 9.8.5 Recent Developments
- 9.9 Vittamed
- 9.9.1 Overview
- 9.9.2 Financials
- 9.9.3 Product Portfolio
- 9.9.4 Business Strategy
- 9.9.5 Recent Developments
- 9.10 Nihon Kohden Corporation
- 9.10.1 Overview
- 9.10.2 Financials
- 9.10.3 Product Portfolio
- 9.10.4 Business Strategy
- 9.10.5 Recent Developments
- 9.11 Others
- 9.11.1 Overview
- 9.11.2 Financials
- 9.11.3 Product Portfolio
- 9.11.4 Business Strategy
- 9.11.5 Recent Developments
- 9.1 Medtronic plc
List Of Figures
Figures No 1 to 37
List Of Tables
Tables No 1 to 77
Prominent Player
- Medtronic plc
- Integra LifeSciences Holdings Corporation
- Codman & Shurtleff, Inc. (Johnson & Johnson)
- Natus Medical Incorporated
- RAUMEDIC AG
- Sophysa Ltd.
- Spiegelberg GmbH & Co. KG
- Vittamed
- Nihon Kohden Corporation
- Compumedics Limited
- Others
FAQs
The key players in the market are Medtronic plc, Integra LifeSciences Holdings Corporation, Codman & Shurtleff, Inc. (Johnson & Johnson), Natus Medical Incorporated, RAUMEDIC AG, Sophysa Ltd., Spiegelberg GmbH & Co. KG, Vittamed, Nihon Kohden Corporation, Compumedics Limited, Others.
The role played by government regulations in the market is substantial as they provide an approving system on medical devices, creation of clinical practice guidelines, determination of reimbursement policies, and medical quality enhancement measures. The regulatory authorities such as the FDA, EMA, PMDA, and NMPA have come up with safety and efficacy standards that a manufacturer has to comply with before the market can be introduced with the devices. The Breakthrough Device Designation program created by the FDA helps both to shorten the development schedule and review schedule of technologies that answer an unmet need. Professional societies such as Brain Trauma Foundation offer clinical practice guidelines that offer evidence-based guidelines on how patients should be selected, which affects their use patterns. Financial coverage depends on the reimbursement policies that were developed by government payers and private insurance companies, and this significantly affects the rates of adoption and market development.
The price of ICP monitoring equipment and the related clinical operations is also a serious limitation of increased uptake, especially in health care systems with limited source bases. The price of invasive monitoring catheters is usually between USD 200 and USD 1,000 per device, and monitoring systems are also another capital expense of USD 10,000 to USD 50,000 or more on the high-end monitoring systems. In addition to the cost of the device, there is the economic cost of the operating room or the entire procedure to insert catheters, intensive care unit costs, specialized nursing care costs, physician professional fees and costs of complications that may occur. Yet, there are a number of factors that are increasing accessibility such as creation of lower-cost single-use sensor technologies, introduction of less expensive non-invasive monitoring devices, rising access to health insurance coverage in developing economies, and the growing awareness of payers that monitoring-based management can result in lower overall costs by achieving better outcomes.
According to the present analysis and forecast modeling, the market of intracranial pressure monitoring devices will witness a significant growth of about USD 3.71 billion in the year 2035 with the increasing indications of monitoring, developments in technology, growing adoption of neurocritical care best practices globally and ongoing clinical evidence of outcome benefits, with a CAGR of 7.4% between the years 2026 and 2035.
It is projected that North America will hold the largest market share in the intracranial pressure monitoring devices market in the forecast period, with a share of about 39% of the global market share, which is attributed to the existence of advanced systems of trauma care, well established neurocritical care infrastructure, high rate and adoption of evidence-based monitoring regimes, well reimbursement systems, market presence of the leading device manufacturers, and high healthcare spending.
The Asia-Pacific Region is expected to be the most growth rate of about 9.5% in the forecast period because of the rising rates of traumatic brain injuries due to the high rate of road traffic accidents, increasing prevalence of stroke and cerebrovascular diseases, extensive government funding in the development of healthcare infrastructure, rising neurosurgical workforce and critical care services, and the size of the population of the countries such as China, India, Japan, and South Korea.
The growing prevalence of traumatic brain injuries and neurological diseases, the growing geriatric population with a higher rate of stroke, the growing popularity of less invasive and non-invasive monitoring devices, and a growing number of neurocritical care facilities globally, the intracranial pressure monitoring devices market expected to continue grow significantly over the forecast period due to the increasing number of clinically relevant clinical advantages of ICP monitoring.
